Hey @constance. Please find response below.
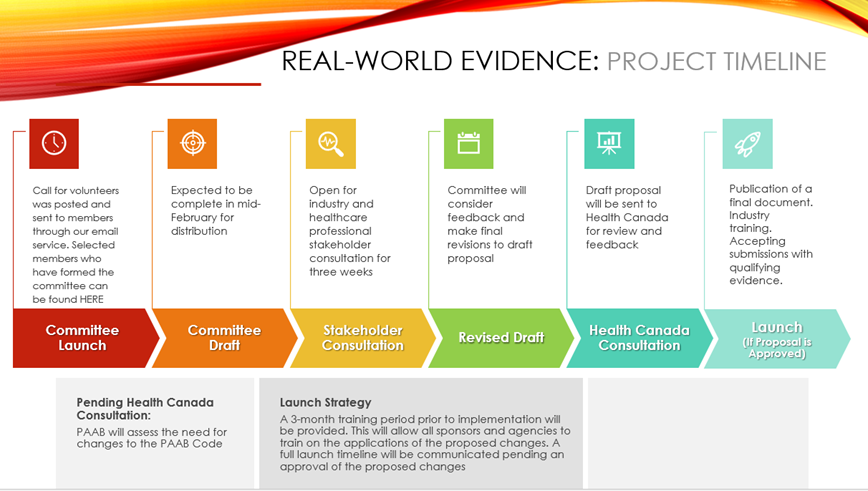
Phase 1 is not complete. There will be rounds of consultation with industry prior to launching to ensure that we are developing as efficiently and in the most supportive manners. Please stay tuned as we look for assistance in finalizing technical validations, user testing, and ensuring adequate support resources are in place. We anticipate these steps will be completed by the end of Q2 and will communicate a go live date as we approach completion.
Manufacturers have been informed about the initiative through communications and the townhall. PAAB has also spoke to this initiative at a number of conferences. We have also invited manufacturers to reach out with specific questions especially surrounding privacy and security. We anticipate that the level of understanding may vary. The purpose of the debrief meeting is to ensure that both agencies and manufacturers have a shared understanding of the initiative while also inviting feedback and suggestions as we develop and scale. As their agencies, your role is pivotal in supporting their implementation efforts, but the debrief aims to equip everyone with the necessary context and clarity to move forward confidently. Please rest assured that we will develop training and guidance related to the new policies and procedures to ensure both agencies and manufacturers are equipped with the knowledge they need to successfully utilize the new features’.
We anticipate that there will be a mixture of manufacturers alone and agencies in collaboration with the manufacturers participating in these meetings. Who participates is at the discretion of the manufacturer. If agencies have agency specific questions, we are also happy to set up these meetings. It may also be possible that the first meeting will be solely with the manufacturer and then they may opt to bring in agencies as it becomes necessary. Please note that currently, these debriefs are not training sessions for the use of new features. These debriefs are intended to discuss security, privacy, and whether/how proprietary manufacturer generated content will be used as we progress through Phase 1 and 2 of the initiative”.
We hope that helps. Thank you for the questions and continued engagement.