Forum Update: Supporting Community-Led Discussion
The forum was created as a space for shared learning and peer support, and as the community grows, we want to lean more fully into that purpose.
Going forward, PAAB will be taking a more listening-first role in forum discussions. Rather than responding immediately to every question, we’ll be encouraging members to engage with one another, share experiences, and help build collective understanding. PAAB will continue to monitor conversations and will step in to:
- Correct any misunderstandings
- Provide guidance when questions remain unanswered after a few days
- Support discussions where official clarification is needed
Our goal is to foster a collaborative, trusted community where knowledge is shared and strengthened by everyone’s contributions.
Thank you for being part of the conversation.
-
-
-
-
-
-
-
-
In celebration of PAAB's 50th Anniversary, Messenger is now FREE of charge with all new and ongoing ARO submissions until March 31, 2026!
Announcements 1
1
-
752 - Hi PAAB, I was reviewing my brands promotional pieces that will need to either be updated or be sent for rePAAB over the next few months. Since some of the pieces were approved, we have had a product monograph update. However, for certain pieces it does not change the Terms of Market Authorization and in some cases the copy in the piece wouldn't need to be changed. The only part of the piece which would need to be changed is the date of the Product Monograph (e.g. brochure) within the references. Since we have significant print inventory, is it permissible to have these submitted as a rePAAB to be valid for the next year until the next update or rePAAB without updating the date of the product monograph within the piece? Please advise. Many thanks.
Claims & Support/References for Claims -
-
-
-
-
-
-
-
-
-
Now Available! Expanded Eligibility for PAAB's Accelerated Review Options (ARO)s
Accelerated Review Options (AROs)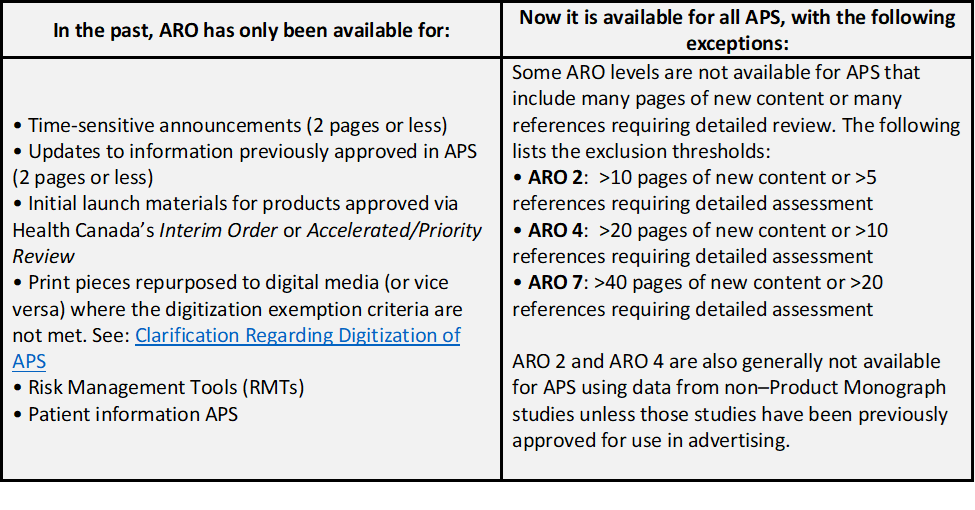 1
1
-
The responses, guidance, and advisories provided by the Pharmaceutical Advertising Advisory Board (PAAB),
including but not limited to those available through the PAAB Forum, the PAAB website, and any PAAB
correspondences, are specifically intended to assist individuals navigating the PAAB preclearance system.
Repurposing or reproducing this content without written consent from the PAAB Commissioner is strictly
prohibited. This prohibition includes, but is not limited to, use in machine learning or AI models.
