Change is in the air for PAAB’s Accelerated Review Options (AROs)!
A client favourite, the ARO service has built a strong track record of expediting approval for the types of submissions that fall within its scope. This success has motivated us to explore creative ways to expand its availability, and make it even better.
This expansion is designed to meet industry needs head-on. We’ve listened to your feedback and invested in processes that make this possible.
The following outlines the key changes to the ARO service including a revised fee structure that will take effect December 8th, 2025.
Historically, ARO was limited to a narrow slice of submission types. As of December 8, 2025 we are flipping the model: ARO is now available for most submissions, with only a few exceptions.
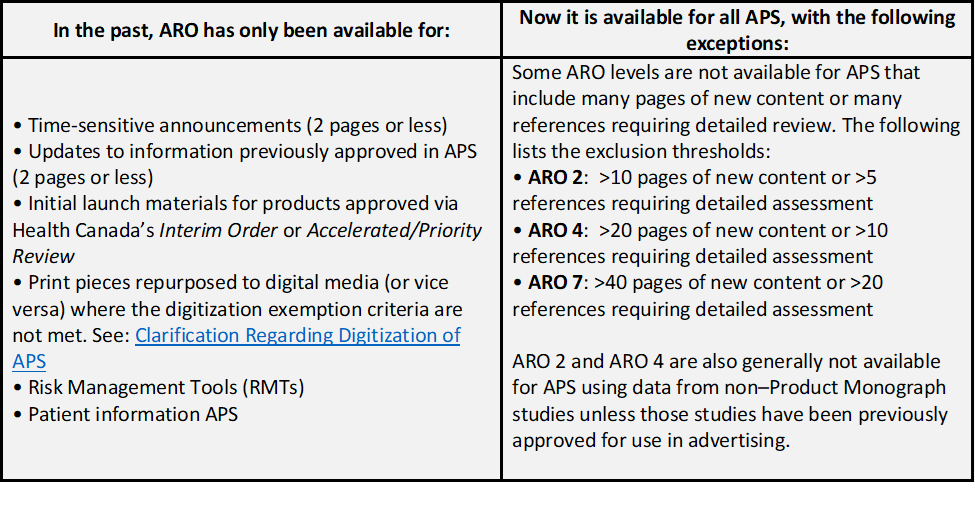
Note: Previously approved content (appropriately shaded) is not considered new content and would not apply to the new content page limits listed above.
If you'd like your APS assessed for ARO eligibility due to special circumstances (e.g., PAAB has already provided an opinion on the acceptability of a study), please reach out to review@paab.ca for a free assessment
We’ve received very positive feedback on the new messenger feature, and early trends show that it effectively contracts time to approval. In advance of our 50th anniversary, we are offering messenger bundled with any ARO review at no additional cost for the end of Q1, 2026.
To activate messenger, either request it within your initial submission form or email review@paab.ca. This applies to both ongoing and new AROs. Please note that only one package of 5 messages is included as part of this offer.
Note: Messenger remains available at cost for standard (non-ARO) submissions.
ARO-2 can now be used for direct-to-consumer (DTC) submissions, provided the submission contains fewer than 10 pages of new content. There are no exclusions applicable to ARO-4.
• ARO supplemental page fee increase to reflect the more detailed assessments that are now available to ARO:
- ARO-7 and ARO-10: $2/page → $4/page
- ARO-2 and ARO-4: $4/page → $8/page
• Removal of automatic upgrades for short ARO submissions: Previously, APS with ≤2 pages of new content received a free level upgrade. This policy created tracking and reporting challenges. Broader ARO availability would further accentuate these challenges. As such, automatic upgrades have been retired.
Please reach out below with any questions.
Thank you
PAAB Team

 ️ PAAB HOLIDAY HOURS
️ PAAB HOLIDAY HOURS  . However, the copy "Now authorized" alone is incomplete (authorized how? for what?) it should be restricted to "Authorized for use in..." or "Authorized for sale in ...".
. However, the copy "Now authorized" alone is incomplete (authorized how? for what?) it should be restricted to "Authorized for use in..." or "Authorized for sale in ...".
 .
.